About
Over 70% of the surface of planet earth is covered by water, with the oceans comprising up to 96% of that water. Although terrestrial plant life has been extensively studied over the years, only in the last few decades with the advent of scuba and underwater submersibles have we began to explore the enormous biodiversity of our planet’s oceans. This vastly under explored realm represents new opportunities for discovering novel natural products with broad applications in the treatment of important clinical conditions.
With the number of antibiotic-resistant bacterial strains continually on the rise, new small molecules are needed in the war against the microbial world. Our group focuses on exploring marine organisms that have historically been understudied (e.g. red alga, sea-weeds, sponges, etc.) toward the discovery of compounds that show activity against various Gram positive/negative bacteria screening programs. Current programs involve the discovery of compounds that show activity in one or more of our screening programs, which include malaria, tuberculosis, both bacterial and fungal pathogens, and known neurological receptors for the treatment pain.
Methicillin-resistance represents one of the earliest discovered instances of anti-microbial resistance (AMR). Methicillin-resistant Staphylococcus aureus (MRSA) quickly became one of the most common test-organisms to analyze the anti-biotic potential of potential anti-microbials. Natural product compounds are routinely tested against MRSA as the first step in the discovery of novel antibiotics, but also to get assist with determining the mechanism of action (MOA) for the test compounds. The anti-MRSA cladophorols are a group of marine natural products isolated from the Fijian filamentous green alga Cladophora socialis by S. Lavoie et al. in 2019. While the activity against MRSA is not impressive for a potential human drug, the activity is not insignificant. The goal of this project is to use a metabolomics approach based on nuclear magnetic resonance (NMR) spectroscopy and liquid chromatography-mass spectroscopy (LC-MS) to determine the anti-MRSA mechanisms of action of the cladophorols. We hypothesize that we can compare the changes in the metabolomes of MRSA to determine if the cladophorols have similar or different MOAs to antibiotics with known MOA.
Related Publications
Lavoie S, Sweeney-Jones AM, Mojib N, Dale B, Gagaring K, McNamara CW, Quave CL, Soapi K, Kubanek J (2019) Antibacterial oligomeric polyphenols from the green alga Cladophora socialis. The Journal of Organic Chemistry. 84 (9):5035-5045. DOI: 10.1021/acs.joc.8b03218
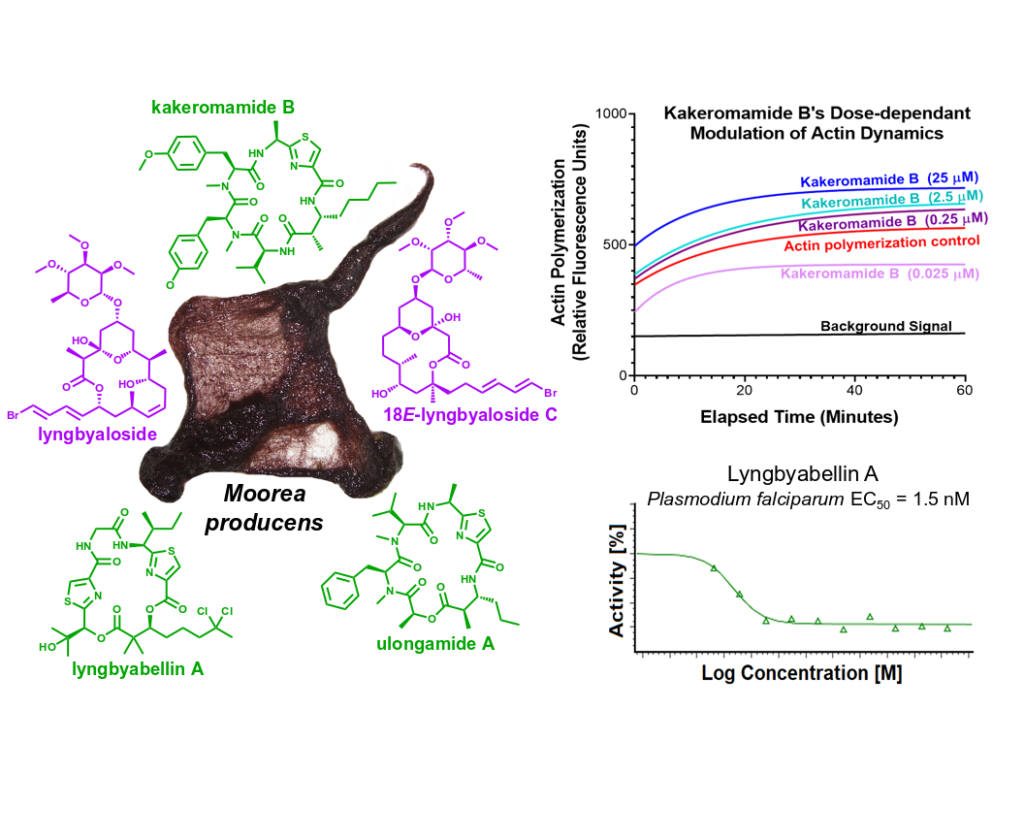
Sweeney-Jones AM, Gagaring K, Antonova-Koch J, Zhou H, Mojib N, Soapi K, Skolnick J, McNamara, C.W, Kubanek, J (2020) Antimalarial Peptide and Polyketide Natural Products from the Fijian Marine Cyanobacterium Moorea producens. Marine Drugs. 18(3):167. DOI: 10.3390/md18030167
Chhetri BK, Lavoie S, Sweeney-Jones AM, Mojib N, Raghavan V, Gagaring K, Dale B, McNamara CW, Soapi K, Quave CL, Polavarapu PL, Kubanek J (2019) Peyssonnosides A-B, unusual diterpene glycosides with a sterically encumbered cyclopropane motif: structure elucidation using an integrated spectroscopic and computational workflow. The Journal of Organic Chemistry. 84(13):8531-8541. DOI: 10.1021/acs.joc.9b00884
Teasdale ME, Prudhomme J, Torres M, Braley M, Cervantes S, Bhatia SC, La Clair JJ, Le Roch K, Kubanek J (2013) Pharmacokinetics, metabolism, and in vivo efficacy of the antimalarial natural product bromophycolide A. ACS Medicinal Chemistry Letters. 4(10), 989-993. DOI: 10.1021/ml4002858
Stout EP, Cervantes S, Prudhomme J, France S, La Clair JJ, Le Roch K, Kubanek J (2011) Bromophycolide A targets heme crystallization in the human malaria parasite Plasmodium falciparum. ChemMedChem. 6:572-577. DOI: 10.1002/cmdc.201100252
Lin AS, Engel S, Smith BA, Fairchild CR, Aalbersberg W, Hay ME, Kubanek J (2010) Structure and biological evaluation of novel cytotoxic sterol glycosides from a marine red alga Peyssonnelia sp. Bioorganic and Medicinal Chemistry. 18:8264-8269. DOI: 10.1016/j.bmc.2010.10.010
Stout EP, Prudhomme J, Le Roch K, Hay ME, Franzblau S, Fairchild CR, Aalbersberg W, Kubanek J (2010) Unusual antimalarial meroditerpenes from Fijian red algae. Bioorganic and Medicinal Chemistry Letters. 20:56625665. DOI: 10.1016/j.bmcl.2010.08.031
Lane AL, Stout EP, Lin AS, Prudhomme J, Le Roch K, Fairchild CR, Franzblau S, Hay ME, Aalbersberg W, Kubanek J (2009) Antimalarial bromophycolides JQ from the Fijian red alga Callophycus serratus. Journal of Organic Chemistry. 74:2736-2742. DOI: 10.1021/jo900008w
Stout EP, Hasemeyer A, Lane AL, Davenport T, Engel S, Hay ME, Fairchild CR, Prudhomme J, Le Roch K, Aalbersberg W, Kubanek J (2009) Antibacterial neurymenolides from the Fijian red alga Neurymenia fraxinifolia. Organic Letters. 11:225-228. DOI: 10.1021/ol8024814
Kubanek J, Prusak AC, Snell TW, Giese RA, Hardcastle K, Fairchild C, Aalbersberg W, RaventosSuarez C, Hay ME (2005) Antineoplastic diterpenebenzoate macrolides from the Fijian red alga Callophycus serratus. Organic Letters 7:5261-5264.